Proteins
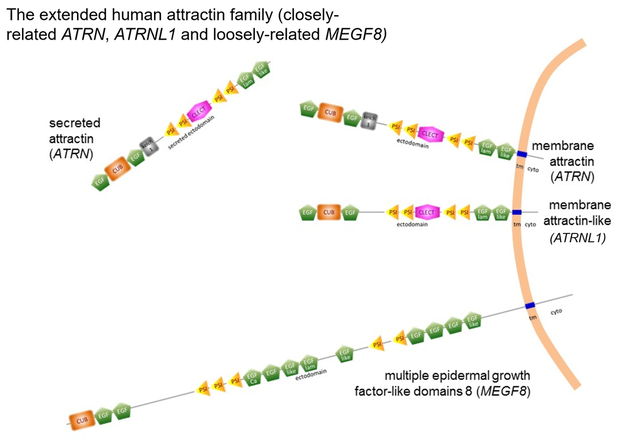
Following the two main transcriptional programs identified in human cells, a Type I transmembrane protein and a secreted protein consisting of essentially the complete ectodomain are translated and have been shown to exist experimentally.
This secreted protein is associated with electron-dense proteoglycan in exocytic vesicles and appears to be released from the cell as these vesicles fuse and open at the plasma membrane. The consequence is that the secreted form appears as a long-lived N-glycosylated protein of at least 180 kDa circulating at levels comparable to complement proteins. A CUB domain with a characteristic β-sandwich fold In the N-terminal region is present, sandwiched between EGF domains. The CUB domain is consistent with attractin's extracellular location and is associated with protein-protein interactions in metazoan organisms. Downstream there are kelch propeller motifs and/or plexin-semaphorin-integrin motifs depending upon one's chosen frame of reference, This region is followed by a C-type lectin domain although sugar-binding has not been demonstrated, a loss of function possibly consequent to variation in consensus residues believed to mediate the calcium dependency important for C-type lectin domain interactions.
The cytoplasmic domain of the transmembrane isoform is almost completely conserved across mammalian species and highly similar to the cytoplasmic domains of fish, birds, reptiles, insects, and amphibians. Within the domain, several peptide motifs are completely conserved across the animal kingdom implying important function in basic intracellular protein-protein interactions.
Interestingly, these cytoplasmic peptide motifs are shared by only 3 proteins, all of which share extracellular domain elements. The transmembrane attractin (isoform 1) is very similar in sequence motif arrangement to attractin-like protein (ATRNL1). The multiple epidermal factor-like domains 8 (MEGF8) protein is more loosely related in the extracellular domain but shares the conserved attractin cytoplasmic domain peptide motifs implying a common ancestor.
This secreted protein is associated with electron-dense proteoglycan in exocytic vesicles and appears to be released from the cell as these vesicles fuse and open at the plasma membrane. The consequence is that the secreted form appears as a long-lived N-glycosylated protein of at least 180 kDa circulating at levels comparable to complement proteins. A CUB domain with a characteristic β-sandwich fold In the N-terminal region is present, sandwiched between EGF domains. The CUB domain is consistent with attractin's extracellular location and is associated with protein-protein interactions in metazoan organisms. Downstream there are kelch propeller motifs and/or plexin-semaphorin-integrin motifs depending upon one's chosen frame of reference, This region is followed by a C-type lectin domain although sugar-binding has not been demonstrated, a loss of function possibly consequent to variation in consensus residues believed to mediate the calcium dependency important for C-type lectin domain interactions.
The cytoplasmic domain of the transmembrane isoform is almost completely conserved across mammalian species and highly similar to the cytoplasmic domains of fish, birds, reptiles, insects, and amphibians. Within the domain, several peptide motifs are completely conserved across the animal kingdom implying important function in basic intracellular protein-protein interactions.
Interestingly, these cytoplasmic peptide motifs are shared by only 3 proteins, all of which share extracellular domain elements. The transmembrane attractin (isoform 1) is very similar in sequence motif arrangement to attractin-like protein (ATRNL1). The multiple epidermal factor-like domains 8 (MEGF8) protein is more loosely related in the extracellular domain but shares the conserved attractin cytoplasmic domain peptide motifs implying a common ancestor.